PAPER ● OPEN ACCESSRead More
1. Introduction
The presented review gives an overview in laser materials processing of energy storage materials and its impact on electrochemical performances and operational lifetime of lithium-ion batteries. Laser ablation of composite electrode materials is a relatively new technology just being validated under laboratory conditions. Laser texturing has different application opportunities regarding tuning of the electrochemical cell performance. It can be applied to each part of the multi-material electrode system, such as the structuring (e.g., drilling, nano-pattering) of the current collector surfaces, selective ablation of the composite electrode surfaces, and structuring of entire composite electrodes down to the current collector. However, a detailed knowledge about the microscopic processes inside composite electrodes and the overall cell system is necessary for enhancing and controlling electrochemical performances via laser texturing. The lithium concentration gradients in liquid electrolytes along the composite electrode induces diffusion of lithium inside the electrolyte. The lithium-ion diffusion kinetics inside the electrolyte filled pores, the charge transfer processes during lithiation and delithiation of active material, the lithium concentration gradients in liquid electrolyte along the composite electrode, and the resulting overpotentials need to be considered in detail. For future battery development, laser processes have a great potential to play a significant role in overcoming diffusion overpotential issues and further engineering challenges of state-of the-art batteries by merging the three-dimensional (3D) electrode and thick film electrode concept.
2. Background
The increasing demand of future batteries with long life-time, low costs, and high power and high energy operation at the same time, needs new electrode or material concepts. Besides the commonly research activities to develop new high-energy materials (“material concept”) based on nickel-enriched NMC (Lithium-Manganese-Cobalt-Oxide) cathodes and anodes made of silicon or silicon/graphite, new electrode architectures, namely 3D battery and mass loading concept, are required in order to push lithium-ion batteries beyond state-of-the-art energy storage concepts.
The mass loading or thick-film concept is about increasing the electrode layer thickness and the active mass deposit in order to achieve a higher gravimetric and volumetric energy density for the entire cell (Figure 1). The standard cell has a NMC electrode layer thicknesses of 50 μm with an areal capacity of 1.39 mAh/cm2. With a cathode film thickness of 125 µm, 250 µm, and 500 µm the specific energy density at cell level can be increased by 18 %, 27 %, and 32 %, respectively. Electrode thicknesses greater than 100 µm can achieve significantly higher specific energy densities at cell level. Increasing the mass loading of NMC electrodes from state-of-the-art 1.39 mAh/cm2 to 10 mAh/cm2 can lead to a 30 % increase in energy density (~190 Wh/kg up to 250 Wh/kg). This corresponds to a volumetric energy density of 582 Wh/L (Figure 1). The thick film concept offers the greatest impact with film thicknesses of up to 300 µm. A further increase in the mass loading would only cause a small increase in the energy density, while the mechanical integrity of the electrodes deteriorates.
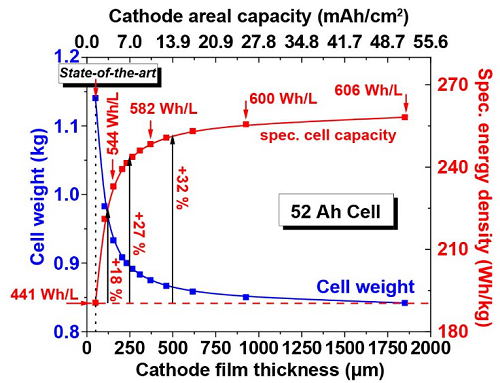
Figure 1. Cell weight, gravimetric and volumetric energy density on cell level as function of film thickness and cathode areal capacity for a 52 Ah cell.
While the energy per area increases with the cathode layer thickness, the power density, or high current capability, of compact layers decreases due to limited lithium-ion diffusion kinetics. Another contribution to elevate overpotential and influence the high rate capability is the length of the path the lithium-ions travel in liquid electrolyte through the composite porous electrode, which is defined by the film thickness, porosity, and tortuosity. Laser structuring of electrodes (3D battery concept) can counteract the drawback of implementing ultra-thick-film electrodes in lithium-ion batteries, namely mechanical stability and high-rate capability. An increased active surface area (i.e., an adapted artificial electrode pore morphology) is suitable to enhance lithium-ion transport in electrolytes, which has a positive effect on capacity retention in a certain range of discharge currents. This 3D battery concept is primarily applied to micro-batteries with applications in micro- and nano-electromechanical systems technology. The design and fabrication of 3D electrodes have become increasingly important in battery systems. Besides laser structuring, advanced 3D printing technologies have promising potential for applications in 3D structured electrodes due to their high design flexibility and capacity for forming complex structures. Cells with structured electrodes show enhanced high-rate capabilities during charging and discharging. They also possess high potential for applications in high-power energy storage devices.
3. Recent Advances
Laser structuring of anodes and cathodes benefits the charge and discharge process, respectively. The literature presents two main strategies to increase the active surface of composite electrodes by laser ablation. One strategy involves drilling of single or double-side coated electrodes; the other approach ablates grids or line patterns with structure depths down to the current collector.
Drilling of electrodes
Recently, it was found that the formation of micro-meter-sized holes on the anodes and cathodes significantly improves the high-rate charging/discharging performance due to the increased number of paths available for lithium-ion transfer to and from the active materials. Matsumoto et al. developed 3D cathodes by drilling the cathode layer and current collectors with a picosecond (ps) pulsed laser. The idea was that through-holes accelerate the lithium-ion transfer in the entire cathodes. Tsuda et al. used a ps-laser to drill electrodes for so-called unbalanced double-side coated LFP (Lithium-Iron-Phosphate) cathodes. The purpose of using unbalanced double-side coated cathodes is related to the fact that thin-film electrodes can sustain a higher capacity for high C-rates (“C-rate” is the ratio of applied electrical current and practical capacity). Figure 2 shows the experimental data and a schematic illustration of the discharging process using through-holed, non-through-holed, and non-holed LFP electrodes. The improvement in the discharging performance of the drilled cathodes at high C-rates results from a facilitated transfer of lithium-ions during discharging through the holes of the LFP electrode. In order to maintain a discharge capacity—at high C-rates and in thick LFP layers—high enough to improve LIB performance, the optimization of the diameter of the holes formed and the number of holes per cm2 are major issues for ongoing research.
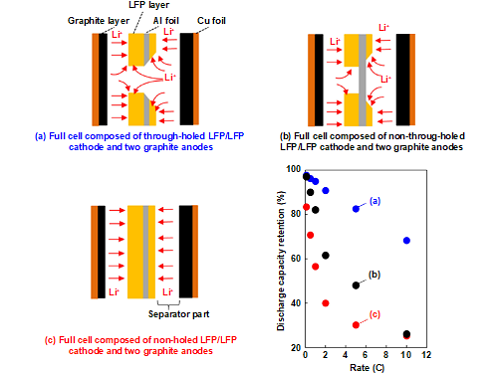
Figure 2. Schematic views of full cells in bi-cell design with different types of laser drilled ((a),(b)) and unstructured (c) unbalanced LFP cathodes and respective discharge capacities as function of C-rate. Reprinted with permission. Copyright 2019 The Electrochemical Society of Japan.
Habedank et al. proposed drilling hexagonal arranged blind holes in graphite anodes. Capacity retention improvements were achieved for C-rates larger than 1C. The strongest impact was obtained for high-power operations and C-rates of about 3C where cells with laser structured anodes provided a 20 % higher capacity compared to cells with unstructured anodes. The observed effect was attributed to reduced diffusion overpotential due to improved lithium-ion transport in liquid electrolyte-soaked anodes.
Line and grid structuring of electrodes
Zheng et al. compared grid and line structures for silicon/graphite (Si/C) electrodes (Figure 3(i)). They concluded that the grid structures were superior to the line structures in terms of isotropic volume expansion and contraction during battery operation. The performance of cells with laser structured graphite or Si/C anodes were compared to cells with unstructured electrodes. As shown in Figure 3(iii), the structured state-of-the-art graphite electrodes achieved impressive performance results with constant capacity values at low (C/5) and high-power operation (3C). This boost in performance can be drawn from the preferential orientation of the graphite flakes, which is parallel to the current collector and perpendicular to the diffusion direction through the electrode. This generates a spatial tortuosity anisotropy and creates particularly long diffusion pathways through the electrode. Due to laser structuring, the preferred diffusion planes are in contact with the free electrolyte, which makes high-power operation possible along the sidewalls of the free-standing micro-pillars.
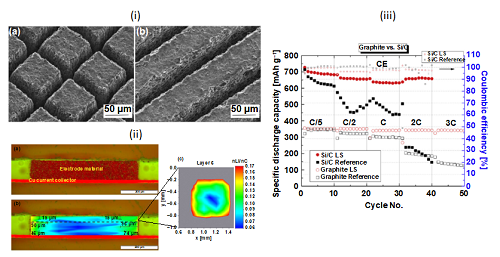
Figure 3. (i) SEM of fs-laser generated (a) grid and (b) line structures in silicon/graphite electrodes. Reprinted with permission. Copyright 2019 Elsevier. (ii) Lithium mapping for illustration of lithium diffusion pathways in fs-laser structured graphite anodes: (a) cross sectional microscopic view; (b) lithium concentration profile in cross sectional view with lithium penetration depths; (c) lithium concentration mapping parallel to the micro-pillar surface. (iii) Specific discharge capacity and coulombic efficiency for cells with laser structured (LS) and unstructured (reference) graphite and Si/C electrodes. Reprinted with permission. Copyright 2019 Elsevier.
Fs-laser ablation has no negative impact on the current collector’s topography or the electrochemical performance, namely the diffusion planes of the active material. The laser ablation enables new diffusion possibilities along the sidewall of the microstructures indicating that no damage of the active material occurs. Concluding from Figure 3(ii), the lithium diffusion length from the sidewall in direction to the center of micro-pillar center is 46-96 µm. This result led to a design rule for anodes that enable optimum lithium-ion diffusion kinetics for high-power operation: the diameter of the laser-generated structures should not be larger than 100-200 µm. The initial capacity of Si/C electrodes exceeded 700 mAh/g, which is twice the value for standard graphite anodes (Figure 3(iii)). The capacity retention for all investigated C-rates remained relatively stable in the cells with laser structured Si/C electrodes. Cells with unstructured electrodes, graphite or Si/C, showed significant capacity fading for C-rate values ≥2C or C/2, respectively. Laser structuring significantly reduced the cell impedance, specifically the charge transfer resistance. This is attributed to a reduced mean tortuosity that facilitated lithium-ion transport and an increased mean porosity, which provides a higher surface area for charge transfer. Furthermore, laser structuring of Si/C electrodes prevents wrinkling, which typically occurs due to substantial volume changes in the thin copper current collector foil.
Structuring of thick film electrodes
Zhu et al. applied the 3D battery concept to Ni-enriched NMC electrodes with a thickness of 250 µm and a mass load of 52-54 mg/cm2 (Figure 4). Using high aspect ratio fs-laser ablation, they reduced the active mass loss to 3 %. For electrodes with a film thickness of 151 µm and 250 µm, laser-structured cathodes with a line pitch distance of 200 µm offer a 40 mAh/g higher discharge capacity at C/2 and 1C, respectively. From lithium elemental mapping it became evident that similar to laser strucutred graphite electrodes additional lithium-ion diffusion pathways are activiated in laser structured electrodes at high-power operation. The sidewalls of free-standing NMC electrode structures show an increased lithium concentration in comparison to the inner part of the so-called micro-pillar model electrodes (Figure 4). A donout-like lithium concentration profile was observed from the top (Figure 4), and a tooth-root-like shape was observed in the cross section. The lithium concentration in structured electrodes reaches values close to the stoichiometric composition of NMC, which corresponds to the safety aspects required for battery operations.

Figure 4. (i) Cross sectional view of laser structured Ni-enriched NMC cathodes with different film thickness. (ii) (a) SEM (top-view) of a laser-structured NMC thick film model electrode for subsequent lithium mapping; (ii) (b) lithium mapping (top view) of a micro-pillar, (ii) (c) lithium mapping (cross sectional view) of micro-pillar after electrochemical characterization.
4. Perspectives
Research in laser structuring of battery materials to improve electrolyte wetting is an approach that, so far, has only been successfully tested at the laboratory scale but shows high economic potential. The next evolutionary step is transferring the process to large-format cells with multiple stacks of electrodes and separators. The improvement of electrolyte wetting in cell production is an approach that can significantly reduce the production costs and failure rates of batteries. Improvements in high-rate capability, due to surface laser structuring, represents another significant up-grade in cell production, especially with regard to increases in electrode mass loading. Laser technology can be incorporated into existing production lines in a modular manner using fiber lasers, scanner technologies, and R2R processes. Scalability to large production quantities is feasible. The structuring process has to be optimized with regard to increasing efficiency and process strategy. A laser system with R2R electrode feeding and high-performance fs-laser radiation meets the technological prerequisites to demonstrate the necessary transfer of laser technology to large-format cells.
5. About the Author

Wilhelm Pfleging is a Senior Lecturer (venia legendi) at university for Laser Technology and Lithium-Ion Batteries and Head of Group Laser Materials Processing at KIT (Karlsruhe Institute of Technology), where he initiates and manages industrial and academic collaborative research and development projects with emphasis on functional surfaces and energy storage systems. He received his Master and Ph.D. degrees from RWTH Aachen (Germany) in 1997. He was research scientist at the Fraunhofer Institute of Laser Technology (Germany) in the field of pulsed laser deposition, laser micromachining, and electron spectroscopy before he joined Forschungszentrum Karlsruhe GmbH as research scientist and principal investigator for Laser Technology. His research focuses on basic and application-oriented research with emphasis on laser-assisted ablation and surface functionalization on micro-/nanometer scale for biological surfaces and microfluidics. In 2008 he started a new research field by merging laser and battery technology. Since 2011 he has been head of the laser material processing group at KIT, where he implemented a new combinatorial approach by setting up an ultra-fast high-energy laser laboratory and a complete manufacturing route for lithium-ion batteries including electrochemical and material analyses. For his pioneering work in the field of “Laser-assisted processes for micro- and nano-scaled structuring and modification of electrode materials” he gained Privatdozent (PD) degree in 2019 He has six patents granted and published over 160 peer-reviewed articles in scientific journals, books and conference reports. PD Dr. Wilhelm Pfleging has given more than twenty of invited presentations and keynote lectures. He took responsibilities as special Issue Editor (Applied Science, SPIE, IJEM) and conference/symposium organizer (MRS, SPIE) and is member in scientific and programme committees in several international conferences (e.g., IEEE 3M-NANO, CLEO, ICALEO, MRS, SPIE Photonics West) with focus on laser processing and energy storage materials.










